carbon valence electrons|Khan Academy : Pilipinas Learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Carbon has two common valences: +2 and +4. See a table of element valences and a printable periodic table. You can scan QR codes on your laptop and your mobile phone. QRscanner.org. Read Image; qr code generator; Scan from camera; Scan from image; QR code scanner. QRscanner.org is an online QR code scanner.It is a perfect tool for scanning QR code online. You can scan QR code from any device like iPhone, iPad, and operating system .
PH0 · What Are Valence Electrons? Definition and Periodic
PH1 · Valences of the Chemical Elements
PH2 · Valence electron
PH3 · Valence Electrons Chart for All Elements
PH4 · The Chemistry of Carbon
PH5 · Khan Academy
PH6 · How Many Valence Electrons Does Carbon (C) Have?
PH7 · 3.10: Valence Electrons
PH8 · 2.9: Valence Electrons
PH9 · 10.6: Valence Electrons
É necessário aderir dentro do jogo. Jogue Lucky 6 Roulette Live pela oportunidade de activar aleatoriamente um Prémio Surpresa. Jogue Lucky 6 Roulette Live e receba pontos tendo por base o seu multiplicador de ganho, para ocupar uma posição na Leaderboard do Torneio de Sexta-feira.
carbon valence electrons*******Learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Carbon has two common valences: +2 and +4. See a table of element valences and a printable periodic table.Valence electrons are also responsible for the bonding in the pure chemical elements, and whether their electrical conductivity is characteristic of metals, semiconductors, or insulators. Metallic elements generally have high electrical conductivity when in the solid state. In each row of the periodic table, the metals occur to the left of the nonmetals, and thus a metal has fewer possible valence electrons than a nonmetal. However, a valence electron of a metal atom has .
carbon valence electrons Mar 23, 2023
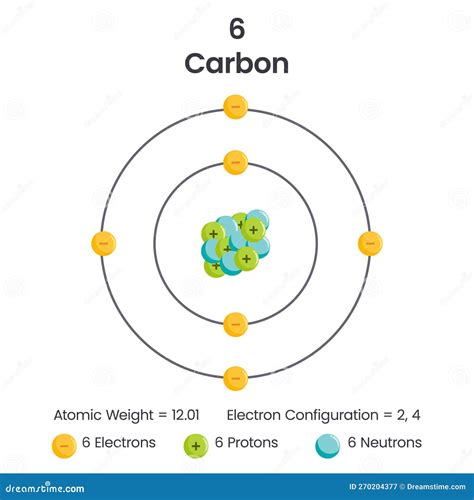
What is the valency of carbon? Ionic properties of carbon atoms. Compound formation of carbon by valence electrons. Easy way to calculate valence electrons in the carbon atom. The valence .Learn what valence electrons are and how to count them for different elements. Watch a video and see examples, definitions, and explanations from other learners and experts. Learn how to determine the number of valence electrons for an element based on its position in the periodic table. Valence electrons are the electrons that .Khan Academy Learn how to determine the number of valence electrons for an element based on its position in the periodic table. Valence electrons are the electrons that .carbon valence electrons Khan AcademyLearn how to identify valence electrons, the outer-shell electrons of an atom that determine its reactivity. See examples for representative and transition elements using abbreviated .
Learn how to identify valence electrons using the periodic table and electron configuration. Valence electrons are the electrons in the highest occupied principal . Learn what valence electrons are and how to find them for any element. Carbon has four valence electrons in its 2s and 2p subshells.Learn about the elemental forms, properties, and compounds of carbon, a nonmetallic element with four valence electrons. Explore the structure and bonding of diamond, graphite, and carbides, and the role of carbon in .
Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from the electron configuration of a carbon atom—1s 2 2s 2 2p 2 —that it has 4 valence electrons (2s 2 2p 2) and 2 core .
The valence electron configuration of carbon atom is 2s 2 2p 2 as shown in the orbital diagram. Figure 1.6g Orbital diagram of valence electrons in carbon atom. Based on the valence bond .
In our example, since carbon is in group 14, we can say that one atom of carbon has four valence electrons. Advertisement. Transition Metals 1. Find an element from Groups 3 to 12. As noted above, the .Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron. Li: 1s 2 2s 1 (the electron in the 2s energy level is the valence electron) Beryllium has two valence electrons: Be: 1s 2 2s 2 (the two electrons in the 2s energy level are the valence electrons) When the carbon atom is excited, then the carbon atom absorbs energy. As a result, an electron in the 2s orbital jumps to the 2p z sub-orbital.. Therefore, the electron configuration of carbon(C*) in excited state will be 1s 2 2s 1 2p x 1 2p y 1 2p z 1.Here, four unpaired electrons exist in the carbon atom.

Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. An atom may give, take, or share electrons with another atom to achieve a full valence shell, the most stable electron configuration.Valence Electrons - Electrons present in the outermost shell that can participate in the formation of a chemical bond or a molecule are called valence electrons. Visit BYJU'S to learn more about Valence Electrons. . Carbon group – Group 14 (IV) 4: Nitrogen group – Group 15 (V) 5: Oxygen group – Group 16 (VI) 6: Halogens – Group 17 . For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. As usual, we will draw two dots together on one side, to represent the 2s electrons. However, conventionally, we draw the dots for the two p electrons on different sides. As such, the electron dot diagram for carbon is as follows: Carbon atoms are always central; b) Connect the central atom with each of the terminal atom by drawing a single bond. 3. For each single bond, subtract two electrons from the total number of valence .Carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in the figure below. Each of these sp 3-hybridized atoms is then .How can you use the periodic table to find out how many valence electrons an element has? This article from Khan Academy explains the simple rules and patterns that can help you determine the number of electrons in the outermost shell of any atom. You will also learn why valence electrons are important for chemical bonding and reactivity.
Carbon (atomic number 6) has six electrons. Four of them fill the 1s and 2s orbitals. The remaining two electrons occupy the 2p subshell. . Valence electrons are also the determining factor in some physical properties of the elements. Elements in any one group (or column) have the same number of valence electrons; the alkali metals lithium . Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second . In the carbon ground-state electron configuration, the two electrons of the 2p orbital are located in the p x, p y orbitals, and the spin of the two electrons is the same. Then the correct electron configuration of carbon in the ground state will be 1s 2 2s 2 2p x 1 2p y 1. Here, carbon has two unpaired electrons. Therefore, the valency of . Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. In cases where an atom has three or fewer valence electrons, the atom may lose those valence electrons quite easily until what remains is a lower shell that contains an octet. 4.5: Lewis Dot and Bonding
To determine the valence electrons of CO 2, it is first necessary to know the valence electrons of the oxygen and carbon atoms. To determine the valence electrons of carbon dioxide we have to follow two steps. It is shown below: Step 1: Determine the valence electrons of carbon and oxygen atoms. The atomic number of .
I used to like him as innn. But I remember nakita ko yan sa isang mall may kasamang ibang girl. That time maingay yung issue na parang may something uli sila ni Angge. Naisip ko pa, baka all for show lang yun since iba kasama ni Carlo sa mall. Tas after nung whole Trina-Carlo-Charlie fiasco, sobrang turned off na ko sa kanya.
carbon valence electrons|Khan Academy